Assay Development
Background
In parallel with the development of gene therapy products there has been the development of clinical assays to gauge the effectiveness of this new therapy.
In many ways this type of research is as demanding as the development of the vectors themselves because without sufficiently powerful assays, it is impossible to determine the effectiveness of our technologies.
The types of assays we have developed can be broken down into a number of groups:
Clinical Biomarkers
Clinical biomarkers are assays that can be performed to give a reliable measurement of the degree of disease severity in a disease.
In CF, spirometric testing of lung function has been a classic biomarker for decades. However, for gene therapy clinical studies we wanted to be able to determine very subtle differences between patients that had received gene transfer vectors and those that had not. This required much more sensitive assays to be developed.
We have looked at a number of areas and you can find publications relating to these below. In particular we have been involved in developing lung clearance index assays (LCI) and believe this may be a more suitable approach to measuring changes in lung function in patients undergoing clinical trials.
Imaging Gene Transfer
One of the most meaningful ways of assessing the effectiveness of gene transfer efficiency is to use direct imaging technologies. For many pre-clinical studies we use fluorescent or luminescent reporter genes to be able to identify the cell types we have transfected in our models.
This allows us to answer one of the fundamental questions regarding gene transfer efficiency, "How many cells are we transfecting and how much do they express?"
Increasingly we are also using direct invivo imaging to make repeated measures of gene transfer in mice or in our ex-vivo models, thus reducing the number of animals and expense involved.
In many clinical trials, antibodies have been used to directly image CFTR protein expression. However, the range of antibodies available is limiting. Nevertheless, our Antibody Working Group has developed a range of improved protocols to optimise such assays in our clinical trial and pre-clinical models.

CFTR protein is shown at the apical surface of the cells (red signal). Cytoplasm labelled with anti Cytokeratin antibody is green and the nuclei are blue (DAPI)
Quantitative RT-PCR
Imaging and biomarkers are very powerful tools to use to assess gene transfer. However, they are not truly quantitative and often fail to detect gene expression from inefficient gene transfer systems.
This can be particularly problematic for early stage projects where maximal sensitivity is desirable. Therefore, the Consortium has a Taqman PCR core facility which is used to detect the presence of vector mRNA or DNA from most of our model systems and clinical trials.
The sensitivity of Taqman is far greater than most other assays available.
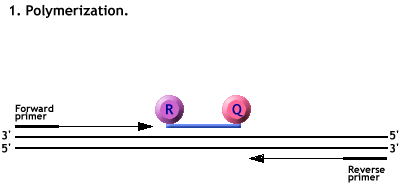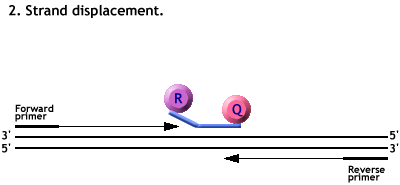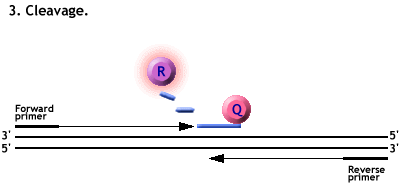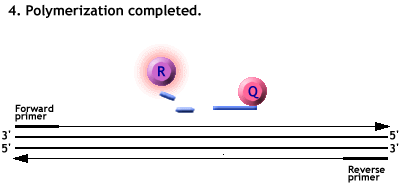
Animation of a Taqman PCR reaction.
Polymerisation from the primers forces the cleavage of the probe.
The Reporter dye is freed from its quencher and fluoresces when excited by a laser
Electrical Measurements of CFTR Function
CFTR forms a chloride channel and passage of ions through this channel can be measured electrically.
We continue to make use of such methods to assess the activity of the CFTR protein following gene transfer.
Publications
